There are more than 26.000 diseases in the world today, of which 7000 are orphan diseases. Orphan drugs are used to treat rare diseases or conditions that affect very few patients among the population ,but can be severe and life-threatening disorders. Although orphan diseases are quite rare, up to 36 million people in the EU alone suffer from such diseases. Worldwide, that number rises to 400 million, though less than 5% of therapies are available to treat them.
Over the past decade, orphan drugs have consistently surpassed the growth of their non-orphan counterparts, showing a promising and stable market. However, the growth rate is slowing down, which raises a few questions like: Why is the growth rate slowing down? And what does the future of the orphan drug market look like?
A Profitable Market
Orphan drugs constitute a profitable niche within the pharmaceutical sector. Studies show that companies integrating orphan drugs into their portfolios are likely to gain greater profitability compared to companies who don’t develop orphan drugs.
One factor is the abundance of rare diseases, which discourages competition in researching treatments for these conditions. Besides the smaller competition, The Orphan Drug Act bolsters this profitability by furnishing incentives like tax credits and up to 50% reductions in research and development expenses for orphan drugs. Moreover, due to the limited patient pool, clinical trials for orphan drugs are typically smaller and less costly. Because the target group is smaller than for more common diseases, companies save a lot of money on marketing strategies and advertisement.
Yet, certain practices have faced criticism. Some pharmaceutical firms explore repurposing treatments already on the market for common diseases to potentially address rare diseases. If successful, the orphan drug designation automatically grants several years of market exclusivity, enabling higher pricing and increased revenues, often with minimal additional costs and research efforts. Today, 70 drugs were first approved by the Food and Drug Administration for mass market use.
Decreasing Growth Rate
Orphan drugs have effectively doubled their market share in global prescription drug sales from 2014 to 2024. Projections indicate that in 2024, they are anticipated to generate a commendable $185 billion in revenue, with estimations suggesting that this figure will surge to approximately $270 billion by the year 2028. Below, the growth rates between 2014-2024 and revenues between 2016-2028 are visualised.
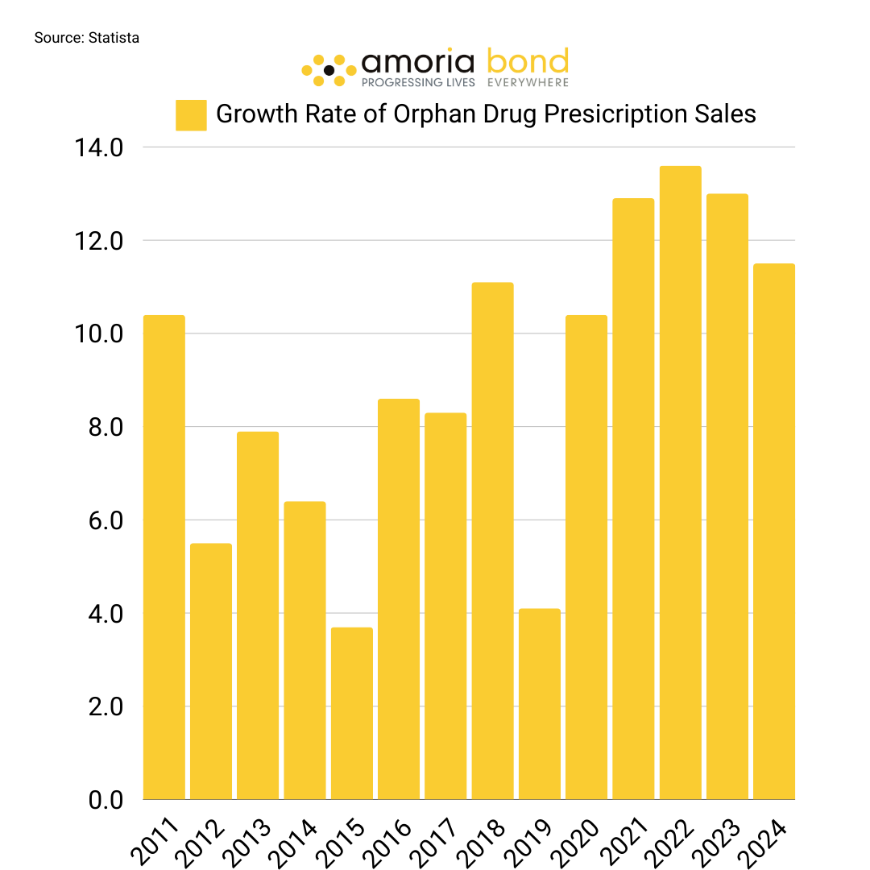
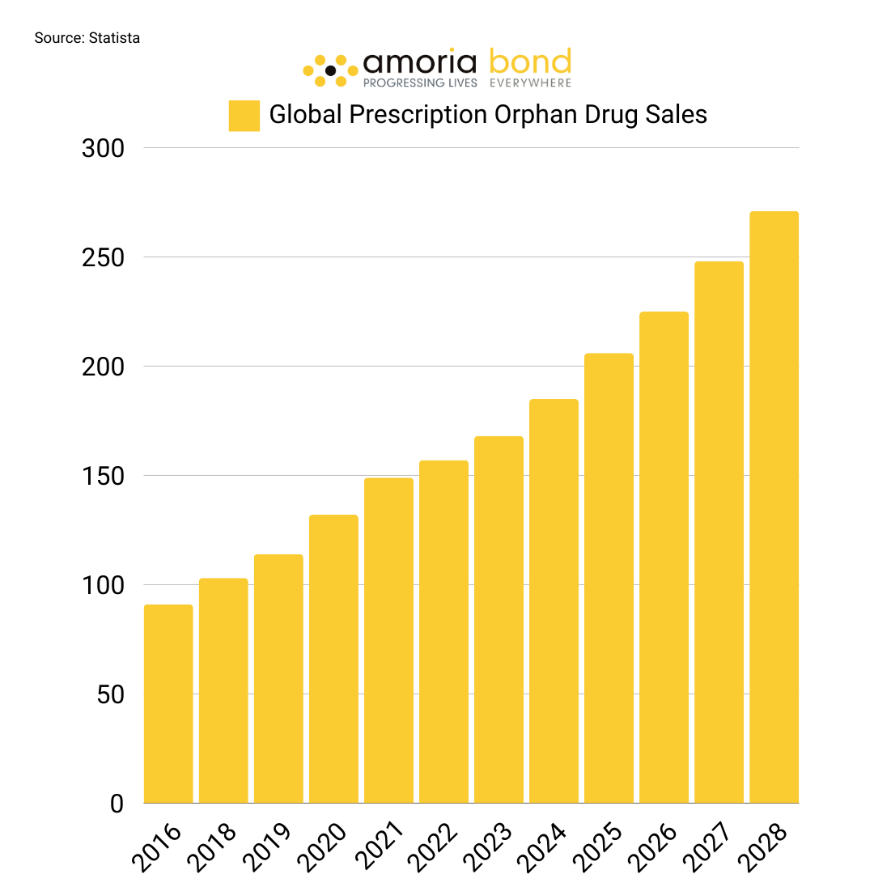
The left graphic illustrates the fluctuating growth rates within the drug market industry. Despite these fluctuations, the market continues to evolve, innovate, and expand, indicating a stable and promising job market. The right graphic further supports this claim by showing higher sales almost every year for nearly a decade.
Both statistics indicate that the global market share of orphan drugs is still growing. However, after three consecutive years of growth rates between 13-14 %, the growth of the market has slightly decreased to 11.5%. By 2030 the growth rate will barely hit two digits. The question arises: why?
Causes of the slowed-down growth rate of orphan drugs
There are several potential reasons why the growth rate of the orphan drugs market is slowing down, including the resurgence of blockbuster drugs for common diseases, as highlighted by a report of Evaluate. While the obesity market currently dominates attention, blockbusters in neuroscience, immunology, and oncology are poised to overshadow the market share of orphan drugs.
Furthermore, the report states that pricing pressures coming from the high costs of orphan drugs add to the challenge. Payers are becoming more selective and reluctant to cover these steep prices, preferring treatments for more prevalent diseases.
Nevertheless, Evaluate's head of thought leadership, Daniel Chancellor, pointed out that the sales slowdown is relative. "As the overall prescription drug market continues to grow, so will that for rare diseases," he stated in a press release.
EU-Regulations and Situation
Creating medications for small patient populations lacks commercial motivation in typical market scenarios. As a result, the EU provides various incentives to foster the advancement of designated orphan medicines. On the website of the European Commission, the following information is provided about legislation for orphan drugs:. Orphan medicinal products target life-threatening or very serious conditions, affecting fewer than 5 in 10,000 people in the EU. Over 200 such medicines are authorised by the European Commission, offering hope to patients with rare diseases.
Sponsors of these medicines enjoy incentives like fee waivers and 10 years of market exclusivity. Additionally, around 2000 products are designated as orphan medicinal products, receiving benefits like protocol assistance to aid their development and approval, ultimately benefiting patients with innovative treatments.
In April 2023, the European Commission introduced two proposals, a new directive and a new regulation, to overhaul EU pharmaceutical legislation. The proposals aim to replace existing laws and streamlining regulations, particularly regarding rare diseases. The Regulation suggests merging orphan drug regulations with general medicinal product laws for simplification and consistency.
The primary objectives include addressing the unmet medical needs of rare disease patients, reducing drug prices for healthcare systems, ensuring equal access to medicines across the EU, and maintaining treatment quality. The proposal aims to redirect investments, promote innovation, enhance EU pharmaceutical industry competitiveness, and alleviate administrative burdens.
An example is that orphan drugs could enjoy up to 11 years of exclusive market rights if they address a significant unmet medical demand. This exclusivity shields them from competition with similar medications targeting similar conditions, which are unable to enter the market during this period.
Overall, the proposal seeks to modernise regulations, improve patient access to orphan drugs, and foster innovation in the pharmaceutical industry while addressing the challenges posed by rare diseases.
What Does The Future Look Like?
Despite the slowed growth rate, the orphan drug market still has a promising future with high revenues and various career opportunities. The market continues to grow, and with new EU regulations, such as longer exclusive rights, orphan drugs are a good investment for pharmaceutical companies, aiding in the treatment of rare diseases.
Another aspect is that only 5% of all rare diseases have available treatments, leading to new research possibilities. As a result, the orphan drug market will remain a lucrative sector with never-ending possibilities.
Looking for a New Job in Pharma?
Our teams are specialised in pharmaceutical recruitment, offering worldwide job opportunities in the field of Life Sciences. We match skilled candidates with employers, who are at the forefront of innovation and progress lives everywhere by developing life-saving medicines.
Are you looking for your next career opportunity in the Pharma industry? Contact us today to find out which job is the perfect fit for you or submit your CV.